What is a datamatrix?
A datamatrix or Data Matrix code is a two-dimensional barcode, also referred to as a 2D-barcode. In the healthcare industry the GS1 DataMatrix is the answer to many issues. This small square symbol is suited for encoding unlabeled products, including very small products such as medicine and medical devices. The datamatrix can also hold additional information such as an expiry date and a batch number.
GS1 DataMatrix in the healthcare, what does it entail?
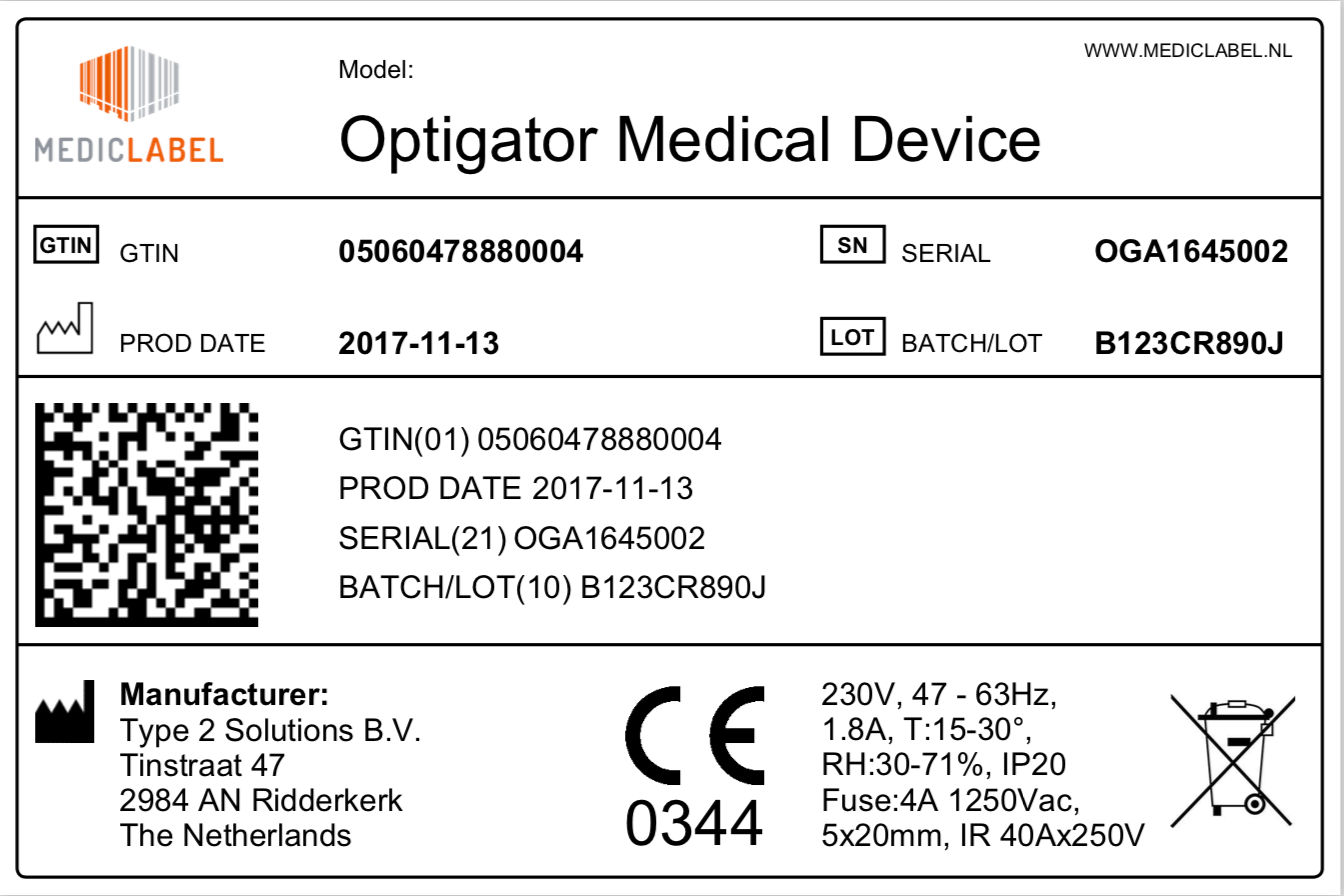 The GS1 Datamatrix in the healthcare is a 2D barcode in which the following 4 components can be encoded:
The GS1 Datamatrix in the healthcare is a 2D barcode in which the following 4 components can be encoded:
- A product identification code: GTIN (01)
- A serial number (21)
- An expiry date (17)
- A batch number (10)
The GTIN (Global Trade Item Number) is the GS1 product identification code. Various international legislations dictate that suppliers assign a GTIN to their products, partly because it’s a worldwide unique number. Depending on the specifics of the legislation a supplier adds a batch number, expiration date and/or a serial number. These components are identified in the barcode through GS1 Application Identifiers (AI’s). These are the numbers shown between brackets, reflecting which encoded information is following.
Unique identification on medicine (FMD legislation)
The Falsified Medicines Directive (EU) 2016/161 (FMD) enters into effect on February 9th, 2019. This means that every medicine packaging, regardless of whether they contain prescription drugs, a unique identification code. This code consists of the following 4 components: a product identification code, a serial number, an expiry date and a batch number. These 4 data elements should be displayed in a GS1 DataMatrix barcode. The goal of this legislation is to counter fake medicine, increase the quality and ensure the integrity of medicines in the pharmaceutical supply chain. It enables tracing each medication from production to the patient.
Unique identification on medical devices (MDR)
Early 2017 the European MDR (Medical Device Regulation) was approved for medical devices. According to the MDR manufacturers of medical devices need to apply a UDI (Unique Device Identifier) to their product. The UDI must contain, at minimum, a unique identification code (a GS1 product number/GTIN). Dependent on the regulations the UDI must also contain a batch, expiration date and a serial number. The identifier must be placed on the product as text and as a barcode, either a linear GS1 128 or a 2D GS1 DataMatrix. The UDI as a barcode enables the scanning of medical devices at hospitals and other healthcare institutions.
Improving patient safety
The introduction of the GS1 DataMatrix is an important step in the continued improvement of patient safety Mediclabel, by Type2Solutions, helps suppliers in the healthcare industry to comply with legislation. Using our Mediclabel Software, you’re able to:
- Assign an identification number (GTIN) to your products;
- Record additional elements such as the batch number;
- Encode the information in a GS1 DataMatrix and / or a GS1 128;
- Generate a Mediclabel including barcode and human readable information to label your products and packages;
- Centrally manage your label related data.
Mediclabel is accessible using a personalized secure web environment. You add your own users and generate an unlimited number of labels.
Experience Mediclabel
Experience the Mediclabel software yourself and generate your own GS1 Data Matrix label quick and easy using our Mediclabel Data Matrix generator demo.
Exchange product data
The GS1 Data Source Healthcare (GDSN) enables suppliers and manufacturers to exchange product data with their customers, such as healthcare institutions. Type 2 Solutions helps healthcare institutions receive this information with the GDSN Mapping Service. This service enables receiving the data in any preferred file format. The data can then easily be loaded into present systems, ensuring all necessary data is at hand to comply with the LIR (National Implants Registry). Want to know more? Go to the GDSN Mapping service website